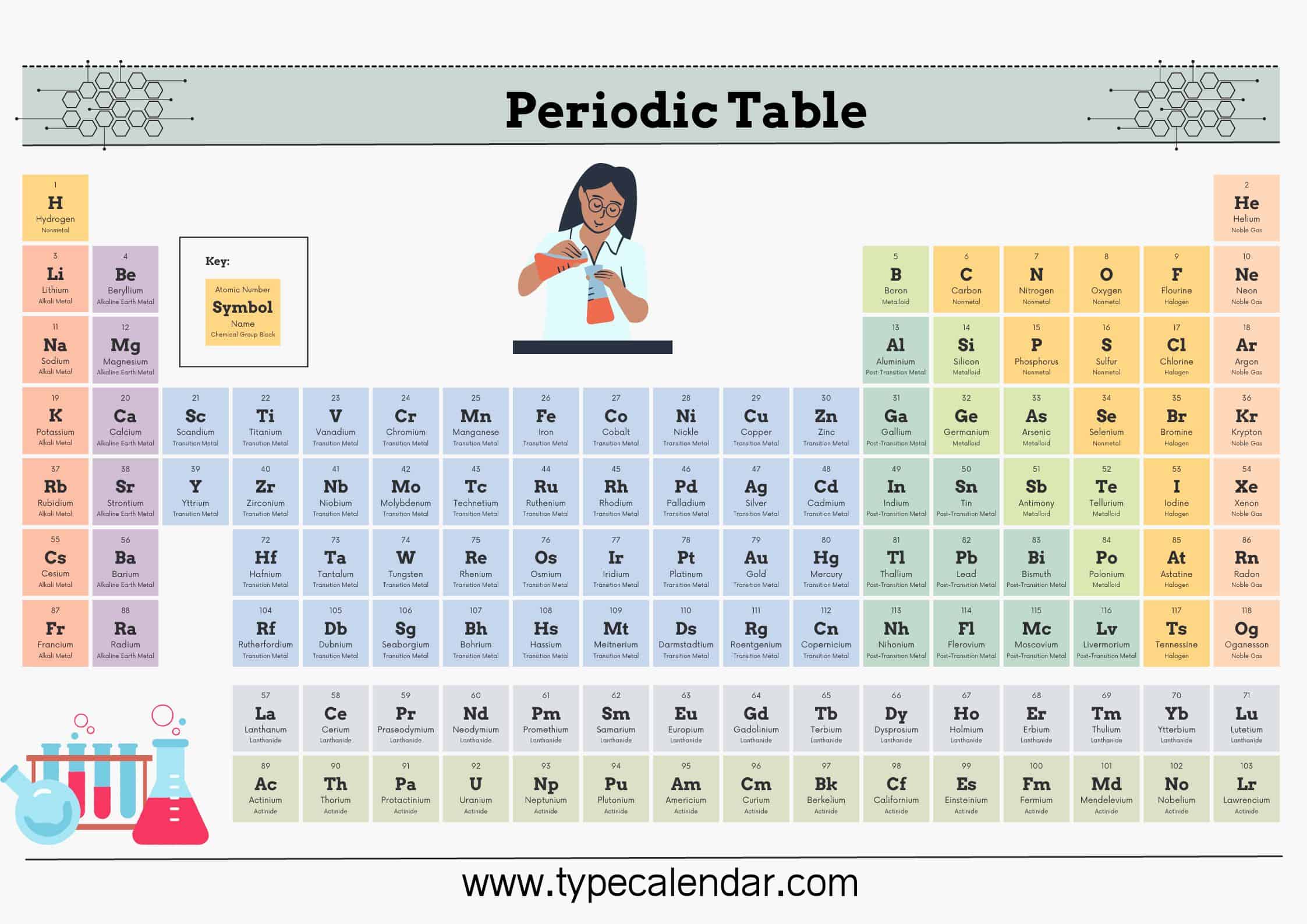
The periodic table is a chart that shows how the 118 known chemical elements are related to one another. The elements are organized into columns and rows. This table was created for your use in homeschooling, science fair projects, or just as a study guide.
Table of Contents
What is the periodic table?

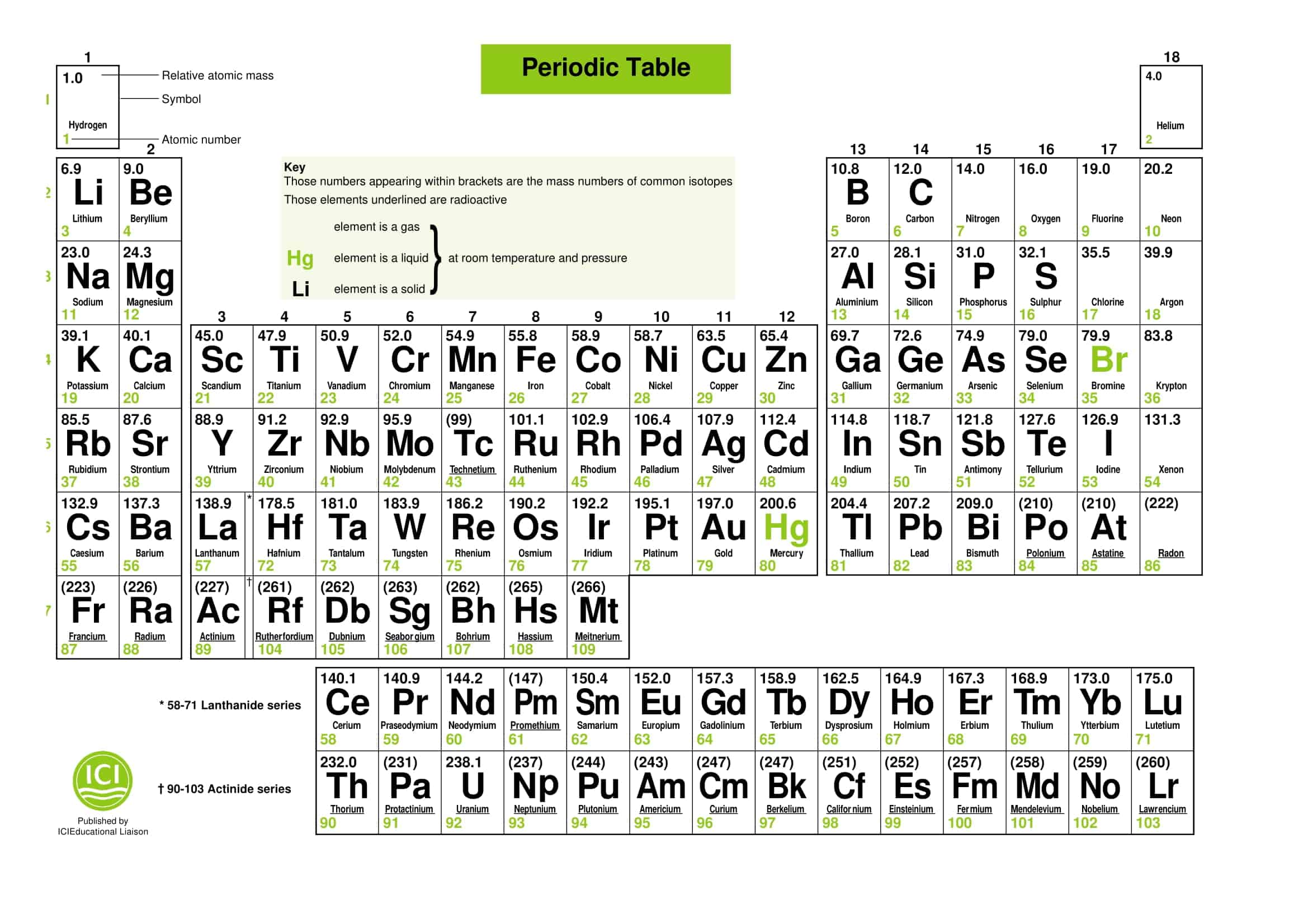
The table developed for classifying chemical elements is called the periodic table. The PERIODIC TABLE, which is also named with names such as the periodic table, the periodic table, and the table of elements, is ordered according to the atomic numbers of the elements.
Periodic Table of Elements with names
Periodic Table Templates are useful tools for studying and organizing the elements. With so many elements, keeping track of their properties can be challenging. Templates provide an organized structure. There are many types of templates to choose from, depending on need.
Some templates focus on the elements’ names, symbols, atomic numbers, atomic weights, and other basic information. These provide a good overview and are handy study guides. Other templates add details like electron configurations, oxidation states, and elemental categories. These give a more in-depth look at the elements’ properties.
More advanced templates may even include specialized information like electron affinity, density, melting point, or reactivity. Templates can be simple and clean or colorful and artistic. There are options for every learning style. Whether needing to memorize key facts or analyze complex trends, Periodic Table Templates are helpful tools for understanding the periodic table. With so many customizable templates available, students can find the right one to meet their needs.
History of the Periodic Table
The periodic table was proposed by the Russian chemist Dmitry Mendeleev in 1869. Using the table, Mendeleev could accurately predict the properties of many elements before they were discovered. Carbon is an essential element for the existence of life. Francium is the rarest element on earth.
There are probably no more than a few ounces on earth at any given time. The only letter not found in the periodic table is the letter J. The country of Argentina is named after the element silver (symbol Ag), which is Argentum in Latin. Although helium is found on earth, it was first discovered by observing the sun.
The periodic table has undergone extensive changes over time since Mendeleev and Moseley originally developed it. Many new elements were discovered, while others were synthesized artificially. The periodic table is the arrangement of elements according to their atomic numbers so that elements with similar properties appear in the same vertical column or group.
The history of the discoveries of the elements is also the history of developments in physics and chemistry.
Before BC, only seven elements were known: gold, silver, copper, lead, tin, iron, and mercury. The smelting of metal ores by fire is one of the greatest revolutions in history. In this way, pure metals began to be obtained from ores in compound form. Again, with the help of fire, alloys could be made by mixing metals.
The idea that fire, air, water, and earth are essential elements dominated human thought for nearly 2000 years.
Thales (624-546 BC) considered water the most basic substance. For Thales, everything came from water.
On the other hand, Anaximander (b. 610 BC) thought that the basic substance was a substance that he called aperion, whose quality was indefinite and whose quantity was infinite.
For Anaximenes (585-525 BC), the most basic (indivisible) substance was air.
Aristotle (384-322 BC) thought that the seven known metals came from the earth, air, fire, and water and that each body was composed of mixtures of these four substances in various proportions.
The most important goal for alchemists was to learn how base metals such as lead or mercury could be converted into gold. For this purpose, they applied various processes to the ores. These efforts of alchemists led not only to the discovery of new elements but also to the invention of chemistry instruments, some of which were used until the 17th century.
The transition from alchemy to chemistry corresponds to the period of study of the properties of air and the discovery of oxygen.
The Elemental Journey
Once upon a time, in a land where elements from the periodic table pdf governed the world, there was a young adventurer named Theodore. He was a curious soul who wanted to learn everything about the elements and their properties. He began his quest by searching for a periodic table with charges to better understand the relationships between the elements.
Theodore discovered that the world was organized according to a modern periodic table pdf. This table was based on an ancient system that had been refined and improved over time. By studying the periodic table of elements printable, he realized that each element had a unique role to play in the world.
With the help of a periodic table printable pdf, Theodore was able to identify and learn about each element. He carried a periodic table with names pdf to assist him in remembering the names of all the elements. As he journeyed further, he started to notice periodic trends in the elemental world.
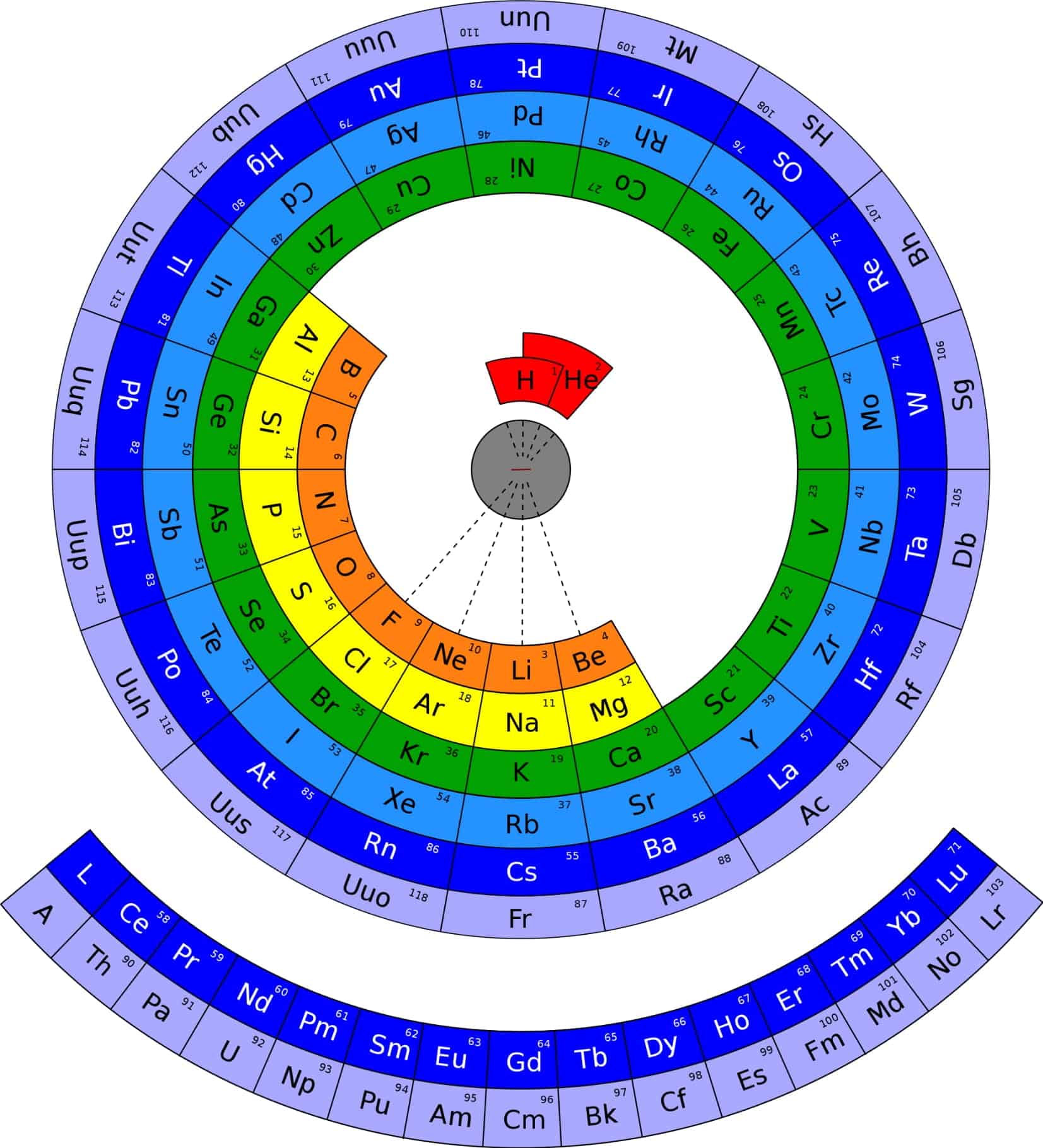
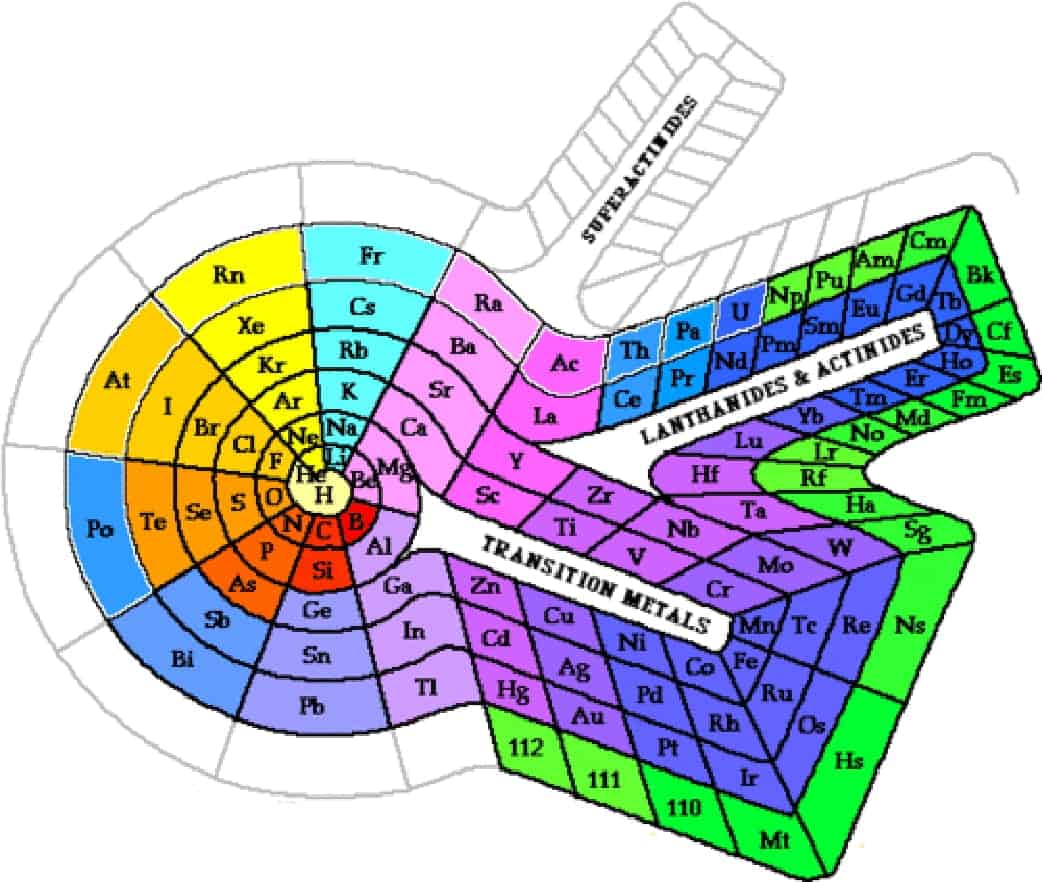
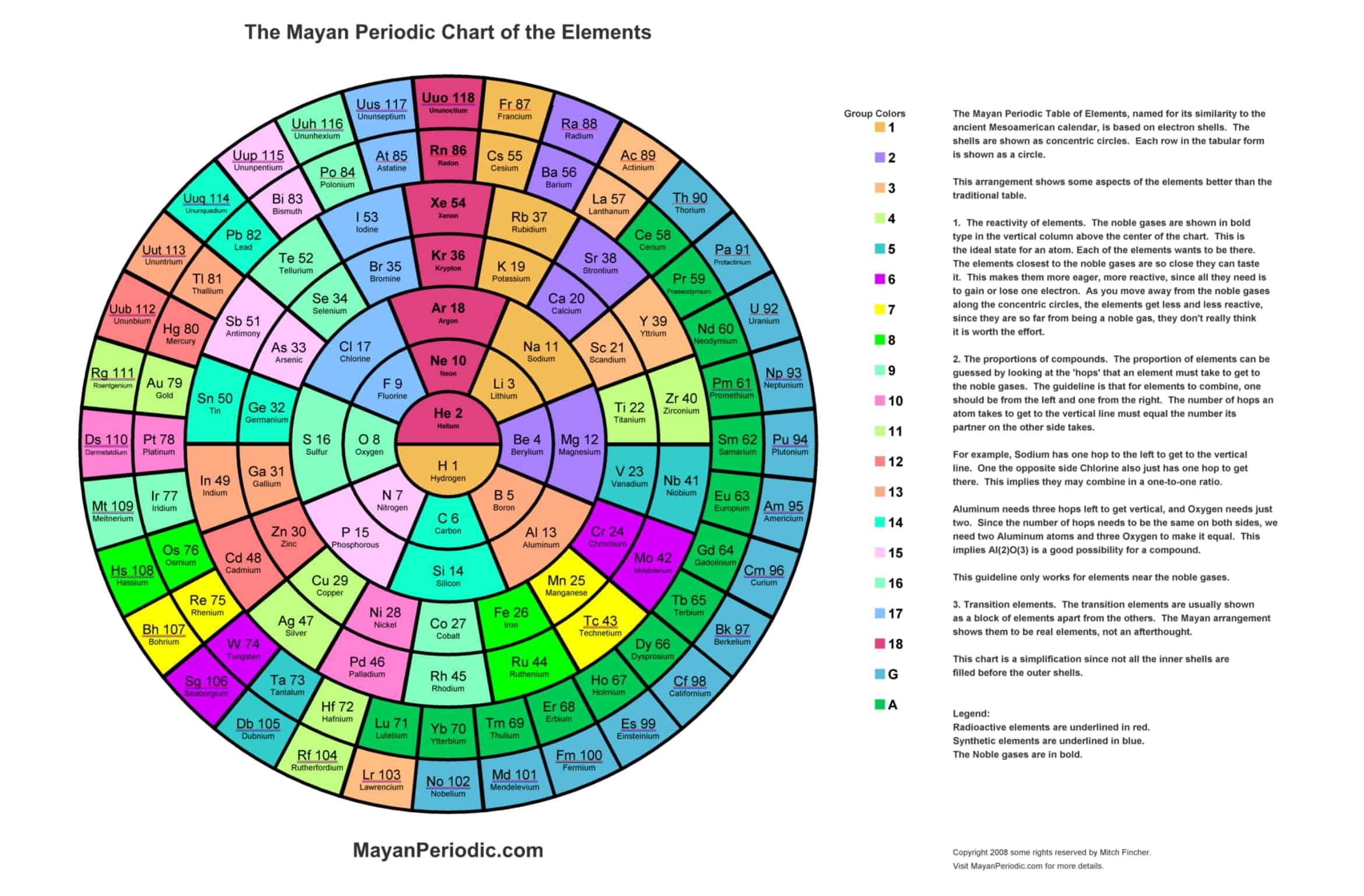
One day, Theodore stumbled upon a mysterious periodic table image. This image depicted a detailed landscape, with each region representing an element. It was a detailed periodic table, unlike any he had seen before. Excited, he used the periodic table pdf with names to navigate his way through this strange world.
Along his journey, Theodore encountered other adventurers who were also on a quest to understand the period table. Together, they discovered a colored periodic table that made it easier for them to visualize the relationships between the elements. With the help of a periodic table print out, they were able to plan their journey more effectively.
The adventurers soon found themselves in a region where the periodic table hd pdf was rumored to be hidden. This rare artifact contained a high-definition representation of the chemistry periodic table pdf, and it was said to hold the key to mastering the elemental world.
And so, Theodore and his friends embarked on a never-ending journey of discovery, embracing the wonders of the elemental world and the knowledge contained within the periodic table. Their quest to understand the elements continued, as they delved deeper into the mysteries of the universe, one element at a time.
How to use a Printable Periodic Table of Elements
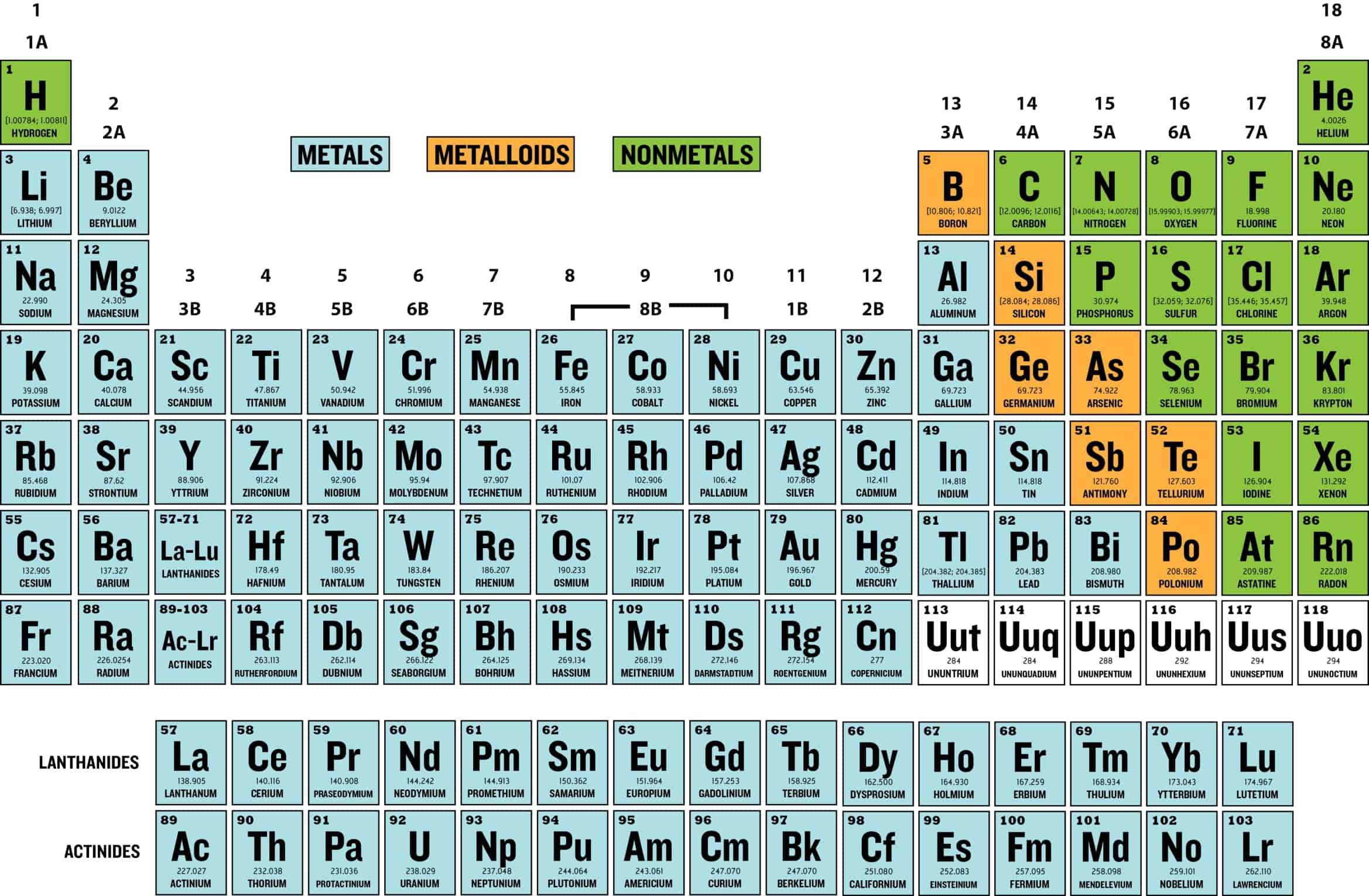
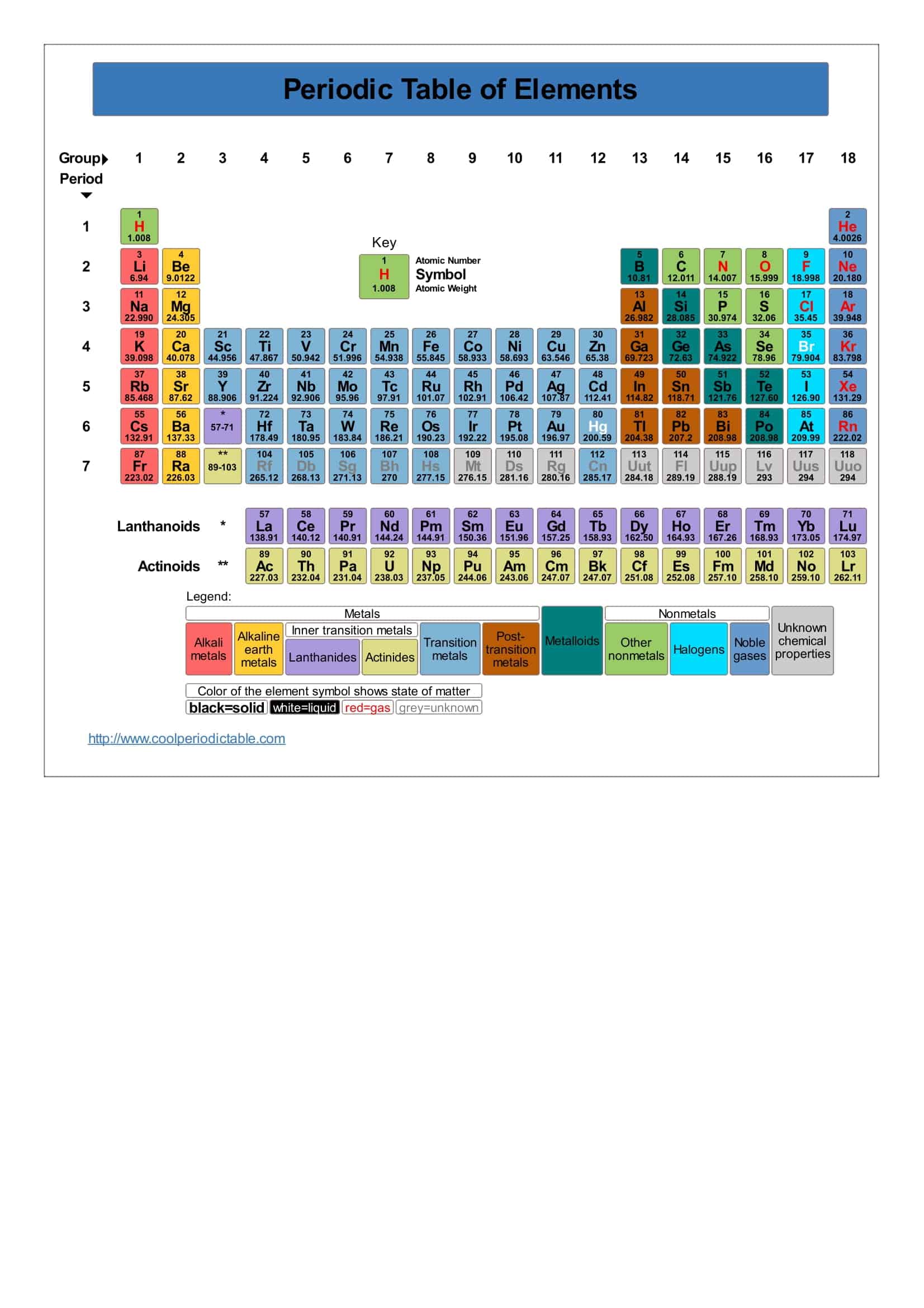
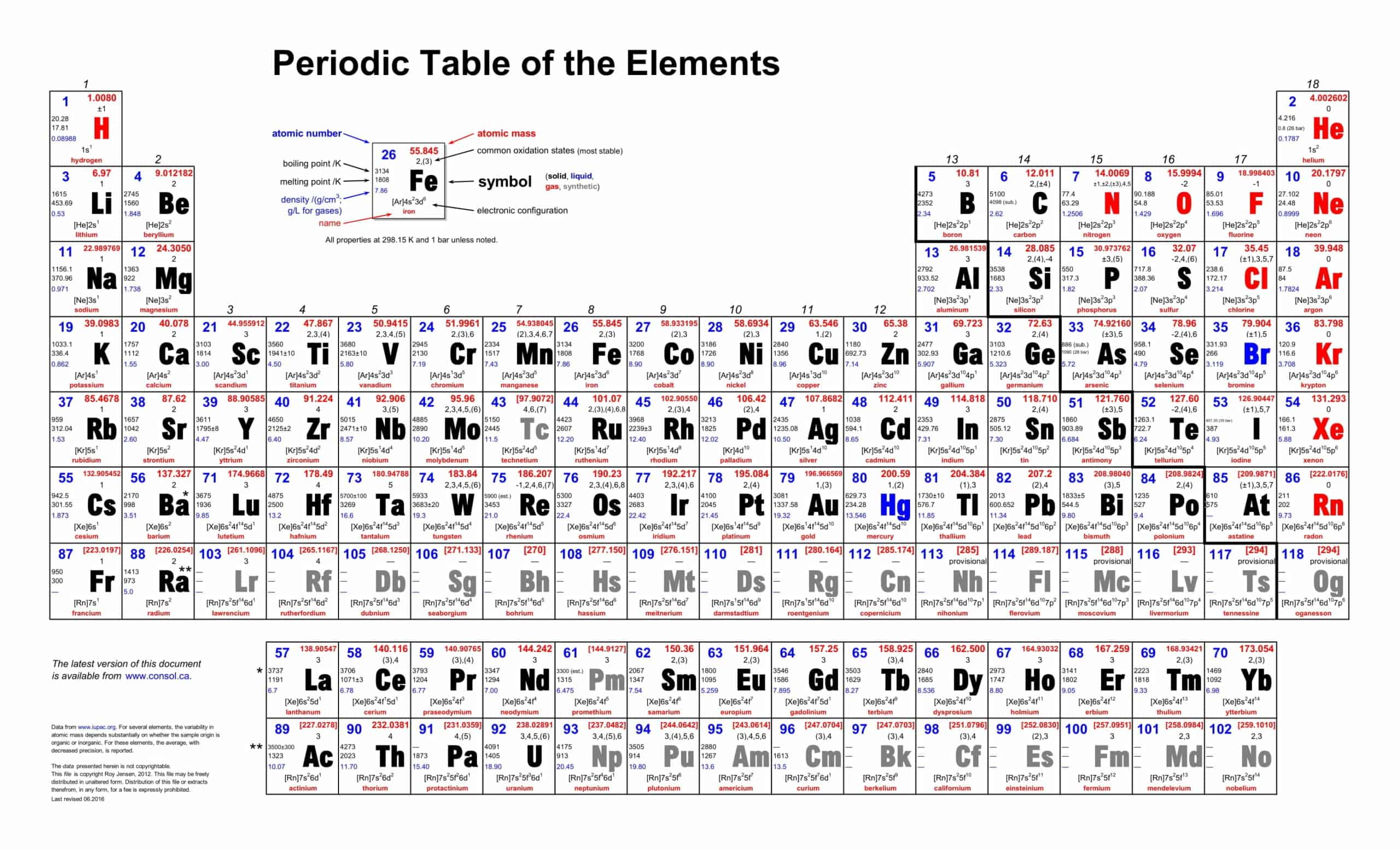
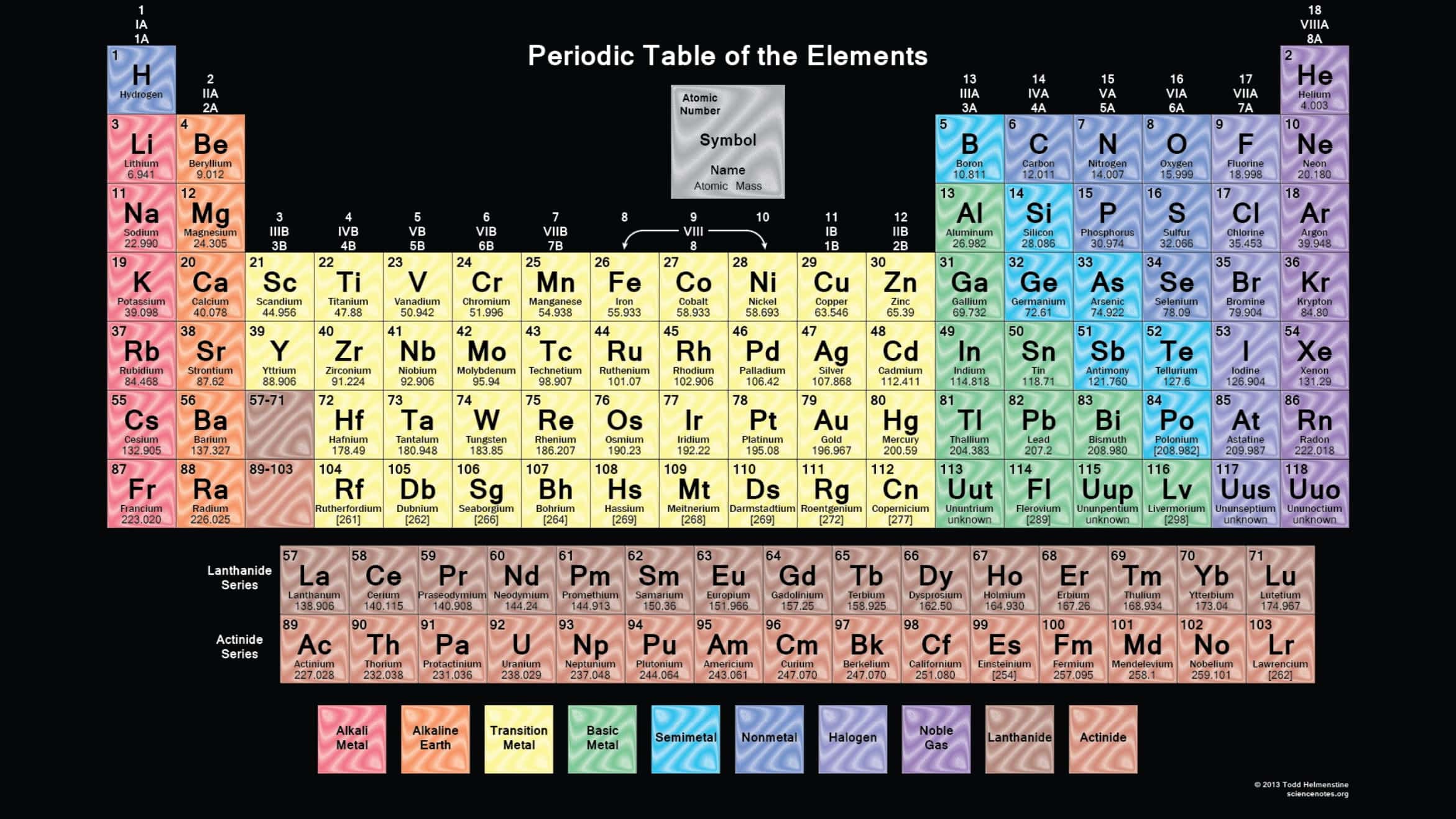
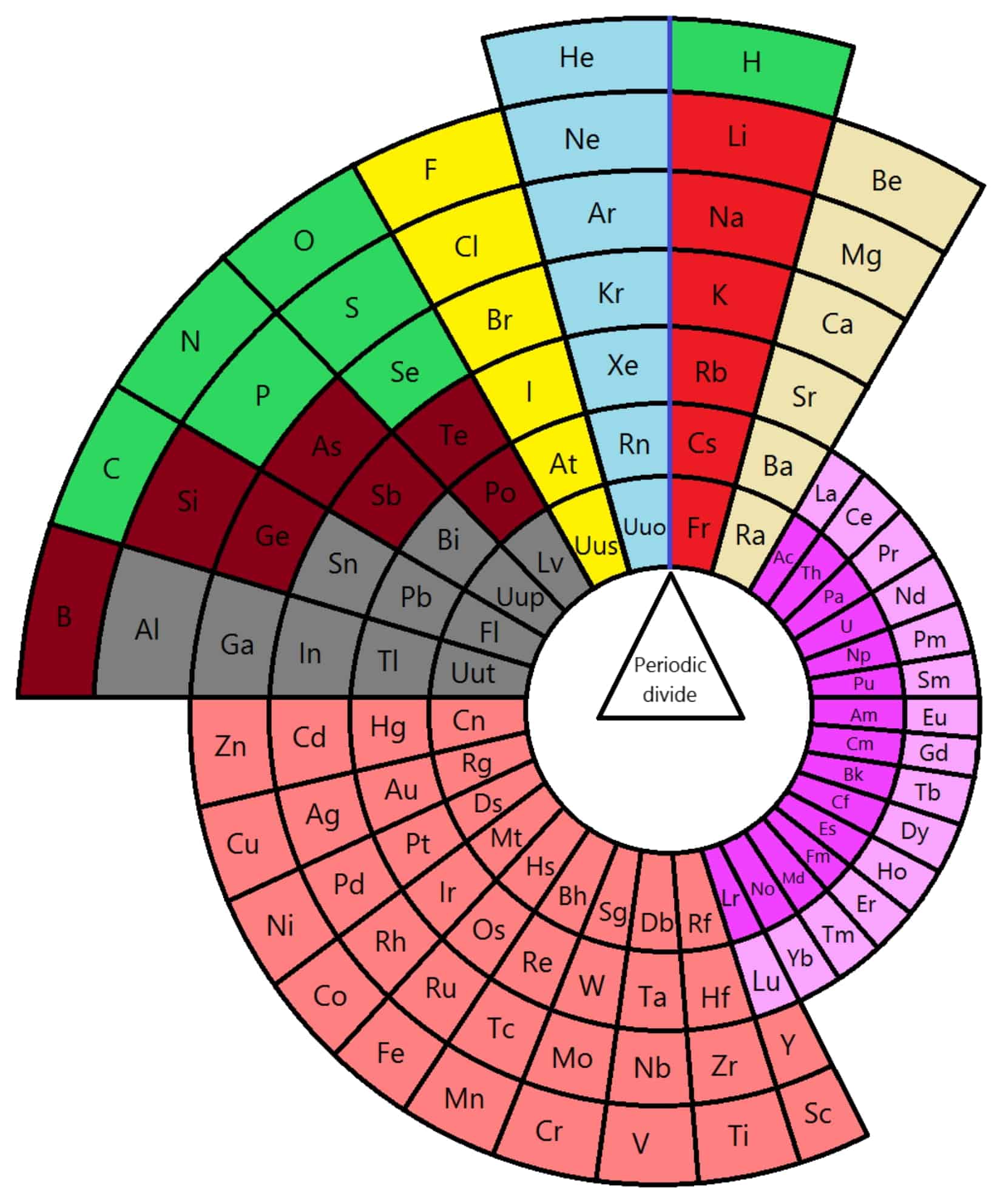
The periodic table is a flexible and user-friendly tool that helps organize the elements. It is divided into cells, which represent an element. Rows are called periods, and columns are called groups. Elements with similar chemical characteristics are given a group name and colored the same color in some tables. There are also four blocks, each containing different groups.
In the modern periodic table, the elements are arranged in order of increasing atomic number.
It is arranged in horizontal rows (period) and vertical columns (groups).
There are seven periods in the periodic table
- 2 in the 1st period,
- 8 in the 2nd and 3rd periods,
- 18 in the 4th period
- 18 in the 5th period,
- There are 32 elements in the 6th period.
- The 7th period is not completed yet.
- The 14 elements of the 6th and 7th periods are placed under the ruler. These are called Lanthanides and actinides.
- Groups are of 2 types, A and B.
- There are 8 A and 10 B groups.
Atoms in the same group have similar chemical properties.
Some of the groups have special names. These names are
- 1A group alkali metals,
- 2A alkaline earth metals,
- 3A group earth metal,
- group 4A carbon group,
- 5A group nitrogen group,
- 6A group oxygen group,
- group 7A halogens,
- Group 8A noble gases and
- B groups are in the form of transition metals.
Exceptions to the Periodic Table;
- Other atoms in group 1A, except the hydrogen atom is, metal.
- Except for the 1st period, the other periods start with the alkali metal.
- Boron(B) in group 3A shows nonmetal properties.
- Fluorine (F) in the 7A group takes only -1 valence in the compounds it makes.
- While the number of valence electrons of the helium atom in group 8A is 2, that of the other atoms is 8.
- Except for Period 7, all periods end with a noble gas.
- A halogen atom comes before other noble gas atoms except helium.
- Transition metals start from the 4th period.
Changing Features On The Periodical Table
- As the month goes from left to right in the periodic table;
- Atomic number increases
- Mass number increases
- Atomic diameter (volume) decreases.
- Metallic character is reduced
- Nonmetallic features increases
- The number of valence electrons increases
- The ability to donate electrons is reduced
- Increases the ability to gain electrons
- The number of protons increases
- Acid properties of oxides of elements increase, and basic properties decrease.
- As you go down the group of the month in the periodic table;
- Atomic number increases
- Mass number increases
- Atom diameter (volume) increases
- Increases metallic character
- The nonmetallic feature is reduced
- The basic feature of the oxides of the elements increases, and the acidic feature decreases
- The number of protons increases
- The number of valence electrons does not change
- The desire to donate electrons increases
- Decreased desire to gain electrons
Conclusion
To summarize, the periodic table is important because it is organized to provide so much information about the elements and how they relate to each other in one easy-to-use reference. The table can be used to predict properties even for elements that have yet to be discovered.
FAQs
Why is element 119 not on the periodic table?
Element 119 has not yet been officially discovered or named. The periodic table includes all elements that have been discovered and confirmed. Once element 119 is synthesized and its properties are verified, it will be added to the periodic table.
How do you memorize the periodic table?
Some tips for memorizing the periodic table include: breaking it down into smaller groups/chunks, associating elements with mnemonic devices or visual imagery, making flashcards to quiz yourself, writing the table out multiple times, using apps or games focused on periodic table memory, and regularly reviewing the table. Focusing on common patterns and trends can help.
Why does the periodic table only go to 118?
The periodic table currently ends at atomic number 118 because this is the heaviest element that has been discovered and verified by scientists so far. Researchers have not yet conclusively created or identified any elements heavier than oganesson (element 118). Theories predict heavier elements may be possible but they would be highly unstable.
How do you write a periodic table?
To write a periodic table, organize the elements by increasing atomic number from left to right and top to bottom in rows called periods. Elements with similar chemical properties are placed in vertical columns called groups or families. Leave empty boxes for elements that have not yet been discovered. Use the elemental symbols and names along with atomic numbers for each box.
How many elements are there in the periodic table?
There are 118 elements in the modern periodic table.
How do you read the periodic table?
The periodic table is read from left to right, top to bottom. Elements are arranged by increasing atomic number. Rows are called periods and columns are called groups/families.
What are groups and periods in the periodic table?
Groups or families are the vertical columns of the periodic table. Elements in a group share similar chemical properties. Periods are the horizontal rows. Elements in a period show trends in atomic structure.
What is the periodic table and how was it developed?
The periodic table arranges chemical elements by atomic number and groups them by their properties. It was developed in the 1860s and 1870s by Dmitri Mendeleev, who left gaps for elements not yet discovered and predicted properties of missing elements.
How are the elements classified in the periodic table?
The main classifications of elements in the periodic table are: metals, nonmetals, and metalloids. Metals are on the left and in the middle, nonmetals are on the right, and metalloids border the stair-step line between metals and nonmetals.





















![Free Printable Credit Card Authorization Form Templates [PDF, Word, Excel] 1 Credit Card Authorization Form](https://www.typecalendar.com/wp-content/uploads/2023/06/Credit-Card-Authorization-Form-150x150.jpg)
![Free Printable Stock Ledger Templates [Excel,PDF, Word] 2 Stock Ledger](https://www.typecalendar.com/wp-content/uploads/2023/08/Stock-Ledger-150x150.jpg)
![Free Printable Financial Projections Templates [Excel, PDF] 3 Financial Projection](https://www.typecalendar.com/wp-content/uploads/2023/05/Financial-Projection-1-150x150.jpg)
